In addition to history and physical, determining the level of medical decision making (MDM) is necessary to define which CPT® code to correctly submit for payment. The American Medical Association's CPT® manual is the authoritative reference for all CPT® related questions and should be available for all physicians to quickly reference. Evaluation and management (E/M) rules are complex and are often open to interpretation, including the high risk MDM component of "drug therapy requiring intensive monitoring for toxicity". The same data presented to different coding experts or auditors will invariably be interpreted differently. It is up to the physician to thoroughly document their thought processes to reduce the possibility of denial of payment during an audit process. The Happy Hospitalist has archived a series of E/M coding lectures through the years to help doctors better understand this complicated process. While I am not a certified coding expert, I have spent years studying the rules and regulations of evaluation and management medicine. Make sure to review all the lectures and the bedside E/M pocket card (picture shown below) to help you with your clinical practice needs.
The Centers for Medicare and Medicaid Services (CMS) has previously published the Evaluation and Management Services Guide. This publication is mandatory reading for any physician wishing to fully understand E&M coding correctly. On pages 16-21, this document provides detailed information on determining risk in the medical decision component of E/M coding. In addition to the three components (history, physical, medical decision making) of E/M, MDM also has three components, the highest two out of three of which are used to determine the overall level of medical decision making. I will in the future be providing a comprehensive discussion on this process. The discussion here shall be limited to understanding the intensive monitoring for drug toxicity of some drug therapy and how it qualifies for high risk medical decision making.
The third component of medical decision making is the risk of complications, significant morbidity and/or mortality, otherwise known as the risk table. The table of risk is presented on on page 20 with the following clarification:
If you came here looking for an absolutely defined, with out a doubt CMS list of drugs allowed for high risk drug management, it doesn't exist. Even CMS states in their discussion of medical decision making that absolutes do not exist. They only provide clinical examples to help guide the clinician in making a determination of what CPT® code to submit for their E&M charge. Some drugs have been identified by the FDA as having a narrow therapeutic index (NTI) which requires intensive monitoring for toxicity. One reference suggested coders use an NTI drug list as their basis for auditing drug therapy requiring intensive monitoring and to add on other drugs physicians felt were warranted. This is a decent starting point. Many coders don't have the clinical experience to understand when some drugs not on an NTI list are high risk in certain clinical circumstances. I believe the most important take home point for physicians is thorough documentation. Documentation is key to helping auditors understand when drug management is and when it isn't high risk. Every patient is different and some drugs may be considered high risk in some clinical situations but not others.
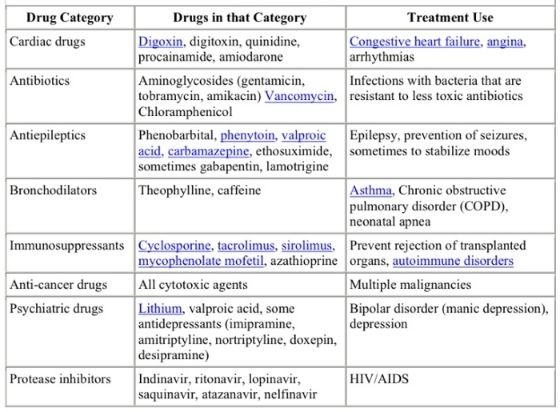
The variability in different opinions from different coders or auditors has roots in the vague E/M language. Drug therapy requiring intensive monitoring for toxicity is a classic example. How does CMS defined intensive? Is hourly monitoring or daily monitoring or weekly monitoring considered intensive? And how is toxicity defined? Does CMS intend to mean toxic drug levels or toxic surrogate side effects? Do they mean complications of treatment? I believe the answers to all the questions can be yes, if given the correct clinical circumstances. One Medicare health administrator, Palmetto GBO, states that cytotoxic chemotherapy is always considered high risk when blood cell counts are used as the surrogate for toxicity. That says to me that drug levels by themselves are not necessary to qualify for high risk drug therapy with intensive monitoring for toxicity. And as far as intensive? What if the CBC isn't ordered until a week after the dose of chemo. Does that meet the threshold for intensive monitoring? Apparently so. This Medicare health administrator provided the following table at the link above with the following clarification. This is the only example on the internet I could find actually presenting a potential list of drugs to be considered for high risk on the E/M risk table.
Even their explanation is vague as they choose not to define "appropriate intervals" for drug levels. My take home point from this list of drugs is we should treat it just as we do using critical care codes CPT® 99291 and CPT® 99292. Just because someone is in the ICU does not mean you can bill critical care and you can bill critical care even if someone is not in the ICU. Just because the drug is on this list does not mean prescribing it qualifies for high risk and just because a drug is not on this list does not mean it isn't considered high risk. Again, I believe documentation is the key to the helping the auditor understand the thought process. This is one more example of how EHR documentation is becoming more about billing and coding for non physician third party payers and less about what is important for communicating a patient's condition to other members of the medical team. Unfortunately, this level of documentation takes time and that time is taken away from face-to-face communication with the patient, another unintended consequence of E/M rules and regulations.
Let's consider warfarin therapy for discussion. Is Coumadin considered drug therapy requiring intensive monitoring for toxicity? As a hospitalist, I take care of a lot of patients on anticoagulation. I believe the answer is yes. Do I directly measure warfarin levels? No I do not, but I do not believe such a lab exists for routine clinical use. The measurement of PT/INR is the clinician's tool for making medical decisions on warfarin therapy. Does the lack of an actual measurement of a drug level exclude Coumadin from the high risk category of MDM? I could find no reference to CMS rules stating only drugs with actual drug level management qualified for drug therapy requiring intensive monitoring for toxicity. Again, I believe aggressive physician documentation is key to help nonphysician auditors understand clinical risk. CMS rules clearly state their intent is to identify conditions which define a high level of risk of significant complications, morbidity and/or mortality.
In the hospital setting, Coumadin use will almost always meet this standard. The potential for excess anticoagulation or under anticoagulation due to drug-drug interactions, under absorption, changes in diet and the constant adjustment in dosing of the medication has the potential to create thrombosis or bleeding complications with catastrophic results. In addition, daily monitoring of Coumadin would most certainly meet the threshold for intensive monitoring. Is there a cut off for how often Coumadin should be monitored to meet the threshold for intenisive monitoring? I don't think a defined time period has ever been established. I think any hospitalized patient on warfarin requiring frequent monitoring (perhaps identified by the hospital's safety protocols) would define Coumadin as high risk drug management because hospitalized patients on Coumadin risk significant morbidity and mortality. If Coumadin use in the hospital is denied by an auditor for not being drug therapy requiring intensive monitoring for toxicity, then I think all drugs should be.
Take the example of chemotherapuetic agents used in cancer. Most certainly these drugs would be considered high risk. But do we actually measure drug levels? Frequently, this type of drug therapy is monitored with electrolyte panels and complete blood counts due to the high risk of electrolyte disturbances, renal failure and bone marrow toxicity from these agents. The Medicare administrator stated above categorically stated cytotoxic chemotherapy was high risk. As I indicated above, if lab data isn't drawn until a week after therapy, does that mean cancer chemotherapy drugs are excluded from meeting the threshold of drug therapy requiring intensive monitoring for toxicity? The Medicare administrator implies no in their discussion linked above. Is it possible to only monitor lab a week after administering a drug and for it to be considered intensive monitoring. It appears so. If chemotherapy agents are excluded from high risk drug management, then I think all drugs should be.
Let us consider a heparin drip. This continuous intravenous medication has a high risk of creating bleeding complications if not monitored with frequent blood draws, often every six hours when first initiated. The monitoring of heparin involves checking partial thromboplastin times (PTTs) or factor Xa levels. Does the failure to monitor actual heparin levels mean this medication cannot be considered drug therapy requiring intensive monitoring for toxicity? If a heparin drip is not considered drug therapy requiring intensive monitoring for toxicity, then no drug should be. The same goes for an insulin drip, amiodarone drip, lasix drip and any other continuous infusion that requires monitoring of a surrogate marker (telemetry, laboratory, radiology).
The list can go on and on. Many drugs may be considered high risk in some clinical situations but not others. How about insulin management in the hospital? We don't measure actual insulin levels, but we do measure glucose to determine whether our insulin dosing is appropriate. Would all hospitalized patients on subcutaneous insulin be considered high risk drug management requiring intensive monitoring for toxicity? One could make an argument that many hospitalized patients are at risk for frequent changes in diet, NPO status, malabsorption, changes in metabolism and many other clinical scenarios that would significantly increase the risk of experiencing complications, morbidity and/or mortality, especially for elderly patients with multiple comorbid conditions.
This is why I think aggressive physician documentation on thought process and analysis of the data is important. In many circumstances, subcutaneous insulin, I believe, deserves to meet the threshold for drug therapy requiring intensive monitoring for toxicity. Is the patient NPO? Do they have an ileus? Are they starting tube feeds? Are they in renal failure? Do they have fever, infection or cardiac issues that are significantly affecting stable blood sugar management and increasing the patient's risk of hyper or hypoglycemic events? Then I believe even subcutaneous insulin should meet the threshold for drug therapy requiring intensive monitoring for toxicity. If insulin management for patient's in these clinical situations do not meet the threshold for high risk drug management, then no medications should.
At the end of this discussion, it should be readily apparent to all that no one list fits all for drugs that can be included in the high risk therapy profile. Because of the failure of CMS to provide clear guidance, I believe the physician has been given a grey area for which thorough documentation will allow work we do every day to be considered high risk and subsequently provide a higher level of E/M coding a payment. The key is for physicians to provide enough documentation for auditors to understand why high risk is present and why the visit meets the criteria of the highest level of medical decision making. If you look closely on my E/M pocket card below, you'll find drug thereapy requiring intensive monitoring for toxicity as one of the reminders to consider high risk for your daily E/M charges.
The Centers for Medicare and Medicaid Services (CMS) has previously published the Evaluation and Management Services Guide. This publication is mandatory reading for any physician wishing to fully understand E&M coding correctly. On pages 16-21, this document provides detailed information on determining risk in the medical decision component of E/M coding. In addition to the three components (history, physical, medical decision making) of E/M, MDM also has three components, the highest two out of three of which are used to determine the overall level of medical decision making. I will in the future be providing a comprehensive discussion on this process. The discussion here shall be limited to understanding the intensive monitoring for drug toxicity of some drug therapy and how it qualifies for high risk medical decision making.
The third component of medical decision making is the risk of complications, significant morbidity and/or mortality, otherwise known as the risk table. The table of risk is presented on on page 20 with the following clarification:
If you review this very important table of risk in the management options selected component on the far right, you'll find drug therapy requiring intensive monitoring for toxicity in the high risk category. This publication by CMS is the basis for defining high risk E/M coding for some drug management. Does CMS help us by defining which drugs are considered high risk and which ones are not? Does CMS provide us with an important list to stay in compliance with our documentation supporting high risk drug management?The table...may be used to assist in determining whether the level of risk of significant complications, morbidity, and/or mortality is minimal, low, moderate, or high. Because determination of risk is complex and not readily quantifiable, the table includes common clinical examples rather than absolute measures of risk.
If you came here looking for an absolutely defined, with out a doubt CMS list of drugs allowed for high risk drug management, it doesn't exist. Even CMS states in their discussion of medical decision making that absolutes do not exist. They only provide clinical examples to help guide the clinician in making a determination of what CPT® code to submit for their E&M charge. Some drugs have been identified by the FDA as having a narrow therapeutic index (NTI) which requires intensive monitoring for toxicity. One reference suggested coders use an NTI drug list as their basis for auditing drug therapy requiring intensive monitoring and to add on other drugs physicians felt were warranted. This is a decent starting point. Many coders don't have the clinical experience to understand when some drugs not on an NTI list are high risk in certain clinical circumstances. I believe the most important take home point for physicians is thorough documentation. Documentation is key to helping auditors understand when drug management is and when it isn't high risk. Every patient is different and some drugs may be considered high risk in some clinical situations but not others.
The variability in different opinions from different coders or auditors has roots in the vague E/M language. Drug therapy requiring intensive monitoring for toxicity is a classic example. How does CMS defined intensive? Is hourly monitoring or daily monitoring or weekly monitoring considered intensive? And how is toxicity defined? Does CMS intend to mean toxic drug levels or toxic surrogate side effects? Do they mean complications of treatment? I believe the answers to all the questions can be yes, if given the correct clinical circumstances. One Medicare health administrator, Palmetto GBO, states that cytotoxic chemotherapy is always considered high risk when blood cell counts are used as the surrogate for toxicity. That says to me that drug levels by themselves are not necessary to qualify for high risk drug therapy with intensive monitoring for toxicity. And as far as intensive? What if the CBC isn't ordered until a week after the dose of chemo. Does that meet the threshold for intensive monitoring? Apparently so. This Medicare health administrator provided the following table at the link above with the following clarification. This is the only example on the internet I could find actually presenting a potential list of drugs to be considered for high risk on the E/M risk table.
"The table below lists examples of drugs that may need to have drug levels monitored for toxicity. This is not an all exclusive list. On medical review, to consider therapy with one of these drugs as a high risk management option, we would expect to see documentation in the medical record of drug levels obtained at appropriate intervals."
Even their explanation is vague as they choose not to define "appropriate intervals" for drug levels. My take home point from this list of drugs is we should treat it just as we do using critical care codes CPT® 99291 and CPT® 99292. Just because someone is in the ICU does not mean you can bill critical care and you can bill critical care even if someone is not in the ICU. Just because the drug is on this list does not mean prescribing it qualifies for high risk and just because a drug is not on this list does not mean it isn't considered high risk. Again, I believe documentation is the key to the helping the auditor understand the thought process. This is one more example of how EHR documentation is becoming more about billing and coding for non physician third party payers and less about what is important for communicating a patient's condition to other members of the medical team. Unfortunately, this level of documentation takes time and that time is taken away from face-to-face communication with the patient, another unintended consequence of E/M rules and regulations.
Let's consider warfarin therapy for discussion. Is Coumadin considered drug therapy requiring intensive monitoring for toxicity? As a hospitalist, I take care of a lot of patients on anticoagulation. I believe the answer is yes. Do I directly measure warfarin levels? No I do not, but I do not believe such a lab exists for routine clinical use. The measurement of PT/INR is the clinician's tool for making medical decisions on warfarin therapy. Does the lack of an actual measurement of a drug level exclude Coumadin from the high risk category of MDM? I could find no reference to CMS rules stating only drugs with actual drug level management qualified for drug therapy requiring intensive monitoring for toxicity. Again, I believe aggressive physician documentation is key to help nonphysician auditors understand clinical risk. CMS rules clearly state their intent is to identify conditions which define a high level of risk of significant complications, morbidity and/or mortality.
In the hospital setting, Coumadin use will almost always meet this standard. The potential for excess anticoagulation or under anticoagulation due to drug-drug interactions, under absorption, changes in diet and the constant adjustment in dosing of the medication has the potential to create thrombosis or bleeding complications with catastrophic results. In addition, daily monitoring of Coumadin would most certainly meet the threshold for intensive monitoring. Is there a cut off for how often Coumadin should be monitored to meet the threshold for intenisive monitoring? I don't think a defined time period has ever been established. I think any hospitalized patient on warfarin requiring frequent monitoring (perhaps identified by the hospital's safety protocols) would define Coumadin as high risk drug management because hospitalized patients on Coumadin risk significant morbidity and mortality. If Coumadin use in the hospital is denied by an auditor for not being drug therapy requiring intensive monitoring for toxicity, then I think all drugs should be.
Take the example of chemotherapuetic agents used in cancer. Most certainly these drugs would be considered high risk. But do we actually measure drug levels? Frequently, this type of drug therapy is monitored with electrolyte panels and complete blood counts due to the high risk of electrolyte disturbances, renal failure and bone marrow toxicity from these agents. The Medicare administrator stated above categorically stated cytotoxic chemotherapy was high risk. As I indicated above, if lab data isn't drawn until a week after therapy, does that mean cancer chemotherapy drugs are excluded from meeting the threshold of drug therapy requiring intensive monitoring for toxicity? The Medicare administrator implies no in their discussion linked above. Is it possible to only monitor lab a week after administering a drug and for it to be considered intensive monitoring. It appears so. If chemotherapy agents are excluded from high risk drug management, then I think all drugs should be.
Let us consider a heparin drip. This continuous intravenous medication has a high risk of creating bleeding complications if not monitored with frequent blood draws, often every six hours when first initiated. The monitoring of heparin involves checking partial thromboplastin times (PTTs) or factor Xa levels. Does the failure to monitor actual heparin levels mean this medication cannot be considered drug therapy requiring intensive monitoring for toxicity? If a heparin drip is not considered drug therapy requiring intensive monitoring for toxicity, then no drug should be. The same goes for an insulin drip, amiodarone drip, lasix drip and any other continuous infusion that requires monitoring of a surrogate marker (telemetry, laboratory, radiology).
The list can go on and on. Many drugs may be considered high risk in some clinical situations but not others. How about insulin management in the hospital? We don't measure actual insulin levels, but we do measure glucose to determine whether our insulin dosing is appropriate. Would all hospitalized patients on subcutaneous insulin be considered high risk drug management requiring intensive monitoring for toxicity? One could make an argument that many hospitalized patients are at risk for frequent changes in diet, NPO status, malabsorption, changes in metabolism and many other clinical scenarios that would significantly increase the risk of experiencing complications, morbidity and/or mortality, especially for elderly patients with multiple comorbid conditions.
This is why I think aggressive physician documentation on thought process and analysis of the data is important. In many circumstances, subcutaneous insulin, I believe, deserves to meet the threshold for drug therapy requiring intensive monitoring for toxicity. Is the patient NPO? Do they have an ileus? Are they starting tube feeds? Are they in renal failure? Do they have fever, infection or cardiac issues that are significantly affecting stable blood sugar management and increasing the patient's risk of hyper or hypoglycemic events? Then I believe even subcutaneous insulin should meet the threshold for drug therapy requiring intensive monitoring for toxicity. If insulin management for patient's in these clinical situations do not meet the threshold for high risk drug management, then no medications should.
At the end of this discussion, it should be readily apparent to all that no one list fits all for drugs that can be included in the high risk therapy profile. Because of the failure of CMS to provide clear guidance, I believe the physician has been given a grey area for which thorough documentation will allow work we do every day to be considered high risk and subsequently provide a higher level of E/M coding a payment. The key is for physicians to provide enough documentation for auditors to understand why high risk is present and why the visit meets the criteria of the highest level of medical decision making. If you look closely on my E/M pocket card below, you'll find drug thereapy requiring intensive monitoring for toxicity as one of the reminders to consider high risk for your daily E/M charges.

Tweet


Tidak ada komentar:
Posting Komentar